 Fracture surfaces of charpy impact specimens showing brittle (left) and ductile (right) fracture (Wadher. R, 05.05.21)
Fracture surfaces of charpy impact specimens showing brittle (left) and ductile (right) fracture (Wadher. R, 05.05.21)
Have you ever wondered why certain metals behave differently in icy conditions compared to room temperature or ambient conditions? At specific temperatures for certain materials, the mechanical properties can change significantly. This phenomenon is known as the ductile to brittle transition temperature. This article will explain this mechanism and how it differs for various materials.
Fracture types can be classified as either being ductile or brittle. Ductile fracture involves a large amount of energy in the process, and this is normally portrayed visually by plastic (permanent) deformation of a sample. Brittle fracture on the other hand requires less energy and propagates much faster than ductile fracture, which fracture surfaces appearing flatter and granular. Therefore, for the purposes of the most applications of metallic materials, ductile failure is desired. However, for certain materials, at a specific temperature there exists a point of no-return where there is a transition in fracture behaviour from ductile to brittle failure. This is known as the Ductile to Brittle Transition Temperature or DBTT. The graph for DBTT for three classes of material is shown below.
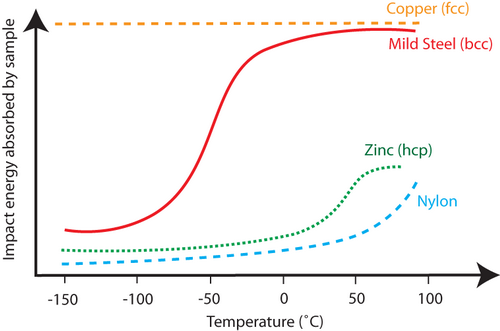 Fig. 1 – Graph showing the different DBTT trends for copper, mild steel, zinc and nylon, (https://www.doitpoms.ac.uk/tlplib/recycling-metals/copper_motors.php accessed 27.04.21)
Fig. 1 – Graph showing the different DBTT trends for copper, mild steel, zinc and nylon, (https://www.doitpoms.ac.uk/tlplib/recycling-metals/copper_motors.php accessed 27.04.21)
As shown in Fig 1, the behaviour of low carbon steels begins to transition at around 0°C whereas low and high strength materials are relatively unaffected by changing temperature. Copper, which has a face-centred cubic (FCC) atomic structure, does not exhibit any DBTT behaviour through a wide range of temperatures. Austenitic stainless steels, such as 316, are also FCC materials and exhibit no DBTT. In the case of Zinc, a hexagonal closely packed atomic structure (HCP), shows a very tight transition range at much higher temperatures and exhibits mostly brittle failure up until 25°C after which it exhibits ductile fracture behaviour. Perhaps most interestingly, Nylon exhibits a drastic change from brittle to ductile behaviour from approximately 50°C and upwards without any gradual transition.
The famous catastrophic failure of the Liberty Ship during World War II highlights the important knowledge and understanding of the ductile to brittle transition temperature. The transition temperature of the metal used on the ship was unfortunately miscalculated during its production. As a result, as the ship sailed into cold-temperature waters, the metal’s behaviour shifted from ductile to brittle. A sudden crack begun to propagate, and eventually catastrophic fracture occurred, cracking the ship in two.
In conclusion, the DBTT phenomenon is highly important behaviour that should be noted when selecting materials for specific applications where temperature sensitivity is of highest importance. It is also important to know that there are ways to effectively “move the transition shelf” to suit the application. For example, methods such as grain size refinement, lowering carbon in the material composition, and a more homogenous microstructure can move the shelf to the left, meaning the material can remain ductile at lower temperatures.
